related sciences
Understanding the surrounding sciences will help you understand the benefits and magnitude of H2.
“Science is a way of life. Science is a perspective. Science is the process that takes us from confusion to understanding in a manner that’s precise, predictive and reliable – a transformation, for those lucky enough to experience it, that is empowering and emotional.” – Brian Greene
water
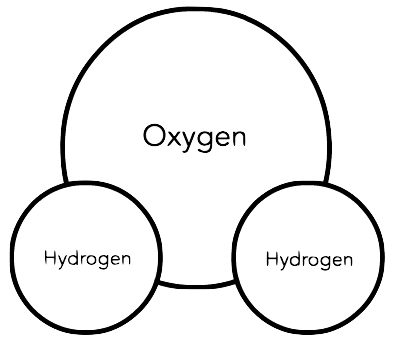
Water consists of two elements: 2 hydrogen atoms and 1 oxygen atom, held together by covalent bonds (sharing electrons).
Oxygen atom: no charge (8 protons and 8 electrons)
Hydrogen atom: no charge (1 proton and 1 electron)
Waters Facts:
- Water is a transparent solvent that can exist in three forms: liquid, solid (ice) and gas (invisible water vapor).
- Water covers 70-75% of the Earth’s surface.
- The human body is 55-75% water
- Water is the universal solvent; no life can exist without it.
- H2O Molecules are held together by (Hydrogen) electrostatic bonds. Water molecules are always in flux, making and breaking bonds with neighboring water molecules very rapidly up to 160 billion times a second. The forces which are being broken are called Van der Waal forces. These are not the same forces as the intra-molecular force holding the H2O together. 1, 2, 3

YOU NEED WATER
Water is possibly the number one thing we take for granted. People use water for every aspect of life: hydration, bathing, cooking, cleaning, etc.
In the U.S. alone, we use about 3.9 trillion gallons of water per month, and the average American uses 176 gallons of water per day 4
In the U.S. a vast majority of people choose sugary, caffeinated drinks over consuming water. These drinks have been shown to increase the risk of metabolic syndrome (obesity, hypertension, dyslipidemia etc). 5
Water is by far the most essential substance for the human body.
The human body is ⅔ water (55% to 75%), which makes it imperative that the body is properly hydrated for good health.
Water percentages, based off the chemistry of the body:
- Brain: approx. = 73%
- Heart: approx. = 73%
- Bones: approx. = 31%
- Lungs: approx. = 83%
- Kidneys approx. = 79%
- Muscle approx. = 79%
- Skin approx. = 64%
- Blood approx. = 90%
Positive effects of being hydrated: 1, 2
- Carries nutrients into the cells and out of the cell
- Lubricates joint and tissues
- Maintains blood volume and viscosity
- Helps to prevent memory loss
- Aids in electrical nerve conductivity
- Helps flush waste and toxins from the body via urine
- One of the primary building blocks of cells
- Helps reduce oxidative stress
- Improves cognitive function
- Acts as a shock absorber for your brain, and other organs
Negative effects of dehydration: 5, 6, 7
- Increased blood pressure
- Increased blood cholesterol
- Increased the risk of coronary heart disease
- Increased risk of kidney disease
- Increased risk of obesity
- Increased risk of type 2 diabetes
- Increased risk of cancer
- Constipation
- Bladder problems
- Increased fatigue
- Increased oxidative stress
- Increased joint pain
- Increased risk of digestive disorders
- Impaired cognitive function
NOTE: Filtered hydrogen-rich water is potentially the best way we can get our daily water intake, for these reasons:
1. Water is essential for life and every function in the human body. 2. Molecular hydrogen is appearing to be therapeutic to the entire human body.
hydration
hydration
The human body is 55-75% water, and proper hydration is a cornerstone of health and wellness. Just drinking more water and getting hydrated will produce many health benefits on its own! The human body is extraordinarily complex and is great at regulating our cellular hydration. It has specific processes that are constant and not dependent on the type of water (High pH, RO, bottled, tap, etc) or liquid it is getting. While hydration doesn’t depend on type of water you consume, there are many things to consider when choosing which water to drink. Remember, hydration is all about consistency, quantity, and quality!

key terms for the science of hydration
Hydration/Cellular Hydration: Hydration for the human body is a key function for life. To be properly hydrated our cells need an ongoing supply of water (H2O). Our bodies have built-in mechanisms to govern our hydration/cellular hydration. 1
Hydration Principles of Physiology: Hydration is governed by blood Osmolarity maintained by the kidneys. This means that the body attempts to regulate how much water it needs depending on the concentration of solutes (electrolytes, proteins, etc.) in the blood. Osmolarity is tightly regulated. If Osmolarity is too high, (over 290 millimoles) cells cannot function properly and we will urinate more frequently and feel less thirsty. If osmolarity is too low (below 280 millimoles) we will urinate less and feel thirsty. 2
Thirst sensation: Is controlled by receptors in the mouth (cells called osmoreceptors) which help regulate the amount of water in the body. Loss of water in these cells will cause you to feel thirstier. 3
Osmotic Gradient: Difference in minerals (mainly potassium and sodium) contained within the cells (intercellular fluid) and outside the cells (extracellular fluid). 5
Cellular hydration: We cannot increase cellular hydration by drinking a different kind of water. All water will hydrate you equally. Cellular hydration is regulated by Osmotic Gradient and Aquaporin Expression. 4
Aquaporin expression: Aquaporins were first discovered in 1992 (6), and a Nobel Prize was awarded for their discovery in 2003 (7). They allow water molecules to enter our cells in a single file fashion. 8
Vasopressin: A hormone that has two primary functions: to retain water within the body and to constrict blood vessels. This hormone also has an important role in cellular hydration. 9
Hypothalamus: The region of the brain right below the thalamus. The hypothalamus regulates multiple activities such as puberty, controlling body temperature, thirst, hunger, and other homeostatic systems, and involved in sleep and emotional activity. 10
antioxidants
antioxidants
Researchers have been looking into how beneficial extra antioxidant supplementation is for the human body. Here is a quote from a study on antioxidant supplementation: “Antioxidant supplements do not possess preventive effects and may be harmful with unwanted consequences to our health, especially in well-nourished populations. The optimal source of antioxidants seems to come from our diet, not from antioxidant supplements in pills or tablets.” 1

Antioxidant
Antioxidants are atoms or molecules with reductive abilities, which means they are able to “donate” or “share” one or more electrons without becoming overactive themselves. They are able to do this because of their conjugated pi system, which allows them to stabilize free radicals by neutralizing them. Antioxidants have the ability excrete anti-inflammatory, anti-aging, and anti-degeneration functions to the human body. The human body has a biological endogenous antioxidant system which includes glutathione, catalase, and superoxide dismutase. These allow the body to offset oxidative stress.
Oxidation
Oxidation is caused by free radicals, which are ions, atoms, or molecules that have unpaired electrons causing them to be very reactive and steal electrons from the closest atom or molecule.
A Free Radical is a reactive atom or group of atoms that can damage cells, proteins, and DNA by altering their chemical structure.
Oxidation occurs throughout the universe and in our bodies. It is an inescapable reaction and a part of aging. In fact, our bodies produce ROS (Reactive Oxidative Species) as a result of cellular respiration. 2-4% of oxygen consumed by the cell’s mitochondria is converted into ROS as a byproduct of cellular respiration. These are free radicals that aid to the aging or breakdown process of the body. When we have an abundance of free radicals in the body it creates oxidative stress.
Oxidative Stress is physiological stress on the body that is caused by the cumulative damage done by free radicals inadequately neutralized by antioxidants
Oxidative stress causes a negative effect on the body and excessive oxidative stress has been linked to numerous diseases and is best balanced with anti-oxidants. 2

OIL-RIG
OIL-RIG is an acronym that will help you to better understand oxidation (free radicals) and reduction (antioxidants): 3
O: Oxidation R: Reduction
I: Is I: Is
L: Loss (of electrons) G: Gain (of electrons)
pH
“P” stands for: A logarithmic function, as well as potential and power.
Think of the “P” as a measurement or amount.
“H” stands for: Hydrogen Ions (H+), which is a another name for a single proton.
The concentration of hydrogen ions (H+) is what dictates a solution’s pH.
Hydrogen ions (H+), are the acidic component of water. Hydroxide (OH-) is the alkaline component of water.
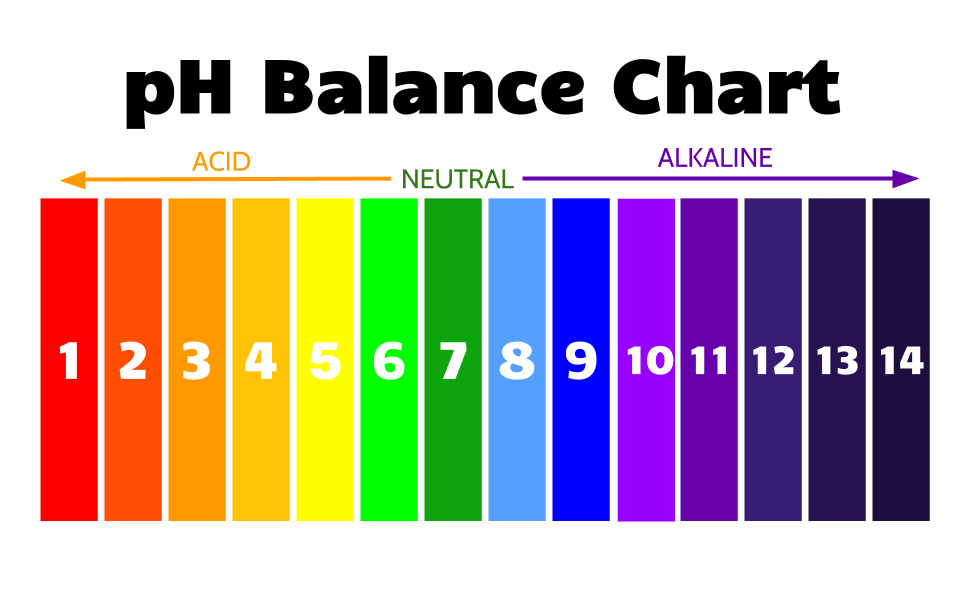
Because pH is the potential of hydrogen ions, the more acidic the solution is, the more hydrogen ions there are, and the more alkaline the fewer hydrogen ions there are.
For example, if I have a glass of acidic water (or orange juice, lemon juice, etc), then there is going to be a higher concentration of hydrogen ions.
If I have a glass of alkaline water, (or bleach, ammonia, etc), then there is going to be a lower concentration of hydrogen ions.
The extra hydrogen ions in acidic water are in the form of hydronium, which is H30+.
The lack of hydrogen ions in water, results in a higher concentration of hydroxide (OH-), resulting in a solution being alkaline. Sine, hydroxide (OH-) is the alkaline component of water, then the higher the hydroxide concentration the more alkaline the solution will be.
Alkaline Water
Alkaline water is water having a pH greater than 7. Water ionizers make alkaline (high pH) water due to the increased hydroxide (OH-) concentration. Hydroxide (OH-) normally exists in these forms, which are called Alkali compounds. These compounds include alkali hydroxides of calcium, magnesium, potassium and sodium.
Water from an ionizer has a high pH but is low in its buffering capacity (ability to buffer acid).
Alkalinity
Alkalinity is the capacity to buffer acid without significantly changing its pH. For example, by adding sodium bicarbonate (i.e. baking soda) to water it will tremendously raise the water’s buffering capacity (alkalinity). For example, 10,000 liters of water from an ionizer at the pH of 10 has the same buffering capacity as one teaspoon of baking soda (NaHCO3). High pH water can be high in its pH but extremely low alkalinity.
Alkalized
Alkalized means to raise the pH of a solution Above 7.
This is a way to describe any process in which the raising of a solution above the pH of 7 occurs.
Examples of Alkalized water:
- Cathodic water from a water ionizer
- Adding ammonia to water
- Adding baking soda to water
- Water treatment facilities that add lye to water 2
pH AND THE HUMAN BODY
Human body’s buffering systems:
- Bicarbonate/carbonic acid buffer system
- Phosphate buffer system
- Plasma protein buffer system
The human body’s blood and organs’ pH are tightly regulated through a set of strong mechanisms known as a buffering system. Blood pH is regulated in a tightly controlled range of 7.35 to 7.45. This range allows for proper cell function, and protein and enzyme activities.
Within the buffering system, the primary drivers are the lungs and kidneys, with secondary drivers being the blood, phosphates, and proteins. These organs and systems have built in mechanisms that helps govern blood pH by removing acids, neutralizing acids with alkaline buffers, or removing alkalis.
Over time the buffering system can become compromised by oxidative stress. This can affect the body’s pH by resulting in 4 conditions:
- metabolic acidosis (non-removal of acid through urine produced by kidneys)
- respiratory acidosis (lungs not removing carbon dioxide (CO2) from the blood)
- metabolic alkalosis (an increase of bicarbonate in the blood)
- respiratory alkalosis (lack of carbon dioxide in the blood)
In light of the human body’s buffering system and the basic understanding of human biology, we know that the body easily adjusts the pH of water. In most instances, the pH of water that is consumed by us doesn’t make it pass the stomach before it is adjusted. If it is not adjusted within the stomach then eventually the water’s pH will be adjusted to 7.4 pH by the blood.
The knowledge of this is important due to the many marketing claims stating that high pH waters can “alkalize” the body or that health benefits can derive from the high pH alone. What makes water vital and critical to the human body is not its pH, but that it is “WATER” and its ability to be the best solvent known to mankind. Meaning it has the ability to carry essential minerals (Na, K, Mg, Ca, etc) into the body, and is the perfect solution for the chemical reactions to take place in the body. Now we know it can carry molecular hydrogen too, a true therapeutic agent. 3, 4, 5, 6
Note: Our bodies are created to have systems in place and these systems work exquisitely without needing any extra help. Our organs each have their own processes that they use to function but need an adequate amount of water and nutrients to function at their best. Maintaining a healthy lifestyle and not going to extremes in either direction will help our bodies and organs to maintain its health. Drinking hydrogen water can help to prevent and protect our bodies from suffering from aging and life-related stress and damage.
ppm
PPM stands for Parts Per Million.
This measurement is similar to 1 out of 100, but it means 1 out of a million. Usually, PPM is used measuring the concentration of something in water or soil.
For example, pertaining to hydrogen water: If we had a 16 oz bottle of hydrogen water measuring at 1 ppm of dissolved of H2, then for every million parts of water, there is 1 part H2.
Based on research, hydrogen water containing 0.5 to 1.6 ppm of dissolved H2 may provide therapeutic benefits to the human body, but PPM’s are not the whole story.
PPM’s = to mg/L (milligrams/Liter)
i.e. 1 ppm/Liter is equal to 1 mg/Liter (33.8 oz)
Preliminary data from research is telling us that 1-3 mg of H2 a day may exert therapeutic effects to the human body.
Figuring your milligrams of H2 a day:
For example: if you drink 1 liter of hydrogen-rich water with a dissolved hydrogen concentration at 1 ppm, then you would have consumed 1 mg of H2.
The higher the dissolved H2 the less volume of water is needed to hit milligrams of H2 per day mark of 1-3mg.
For example: if you drink 1 liter of H2 water with a hydrogen concentration at 3 ppm, you would have consumed 3 mg of H2. This means that you have reached the goal of 1-3 mg of H2 for the day with only 1 liter of water.
Note:
1-3 mg is not the minimal amount needed to provide benefits from ingesting H2. 1-3 mg simply appears to be the amount that is generally used in human studies, which does show therapeutic effects.
There are many factors (e.g. size, genetics, diet, etc.) and in many cases diseases, in which the amount of H2 needed may be higher or lower.
For more information visit:
