Hydrogen inhalation is one of the most common—and most researched—methods of administering molecular hydrogen (H₂) for therapeutic use [1]. Peer-reviewed studies have consistently shown that inhaling hydrogen gas can deliver systemic benefits, with applications ranging from oxidative stress reduction to potential neurological and cardiovascular support [2][3][4]. This is true not only in controlled clinical research but also in the therapeutic hydrogen industry, where inhalation devices have become increasingly popular.
Following the COVID-19 pandemic, interest in hydrogen inhalation devices surged worldwide. Hospitals in Asia, particularly in China and some other countries, reported promising outcomes when using H₂ therapy as part of recommended adjunctive therapy[5][6]. This clinical attention sparked a rapid increase in consumer demand, leading to a rise in hydrogen inhalation products entering the market. Today, dozens of companies sell a wide range of H₂ inhalation devices—each promoting their own models as “the best.”
But this raises a critical question: Which hydrogen inhalation device is truly the best, and what should consumers actually be looking for?
The Problem: Bias in the Market
Here’s where things get tricky. Every company in the hydrogen space has something to sell, and when your bottom line depends on your product, it’s almost impossible not to be biased. That doesn’t mean every company is necessarily lying or malicious; it simply means they view their own hydrogen devices through a lens that makes them look like the superior option every time.
For example, companies that build and sell oxyhydrogen devices will almost always insist that Brown’s Gas is the most effective form, citing reasons they believe support that position. Companies selling devices that mix hydrogen with air will argue that their units are the most safe, balanced, and practical method. And of course, companies producing pure H₂ inhalation units will argue that their devices are the safest and most low maintenance options. Each side has evidence, stories, and reasons that seem compelling—but each perspective is inevitably shaped by business interests, even if there are some kernels of truth in each one’s sales pitch.
This dynamic leaves consumers stuck and confused. Some people become paralyzed by the conflicting information and don’t buy anything at all. Others plant a flag with one camp and defend it no matter what, often overlooking legitimate limitations of that technology. Neither of these outcomes serves the consumer well, and both contribute to confusion in the market.
Why H2HUBB Exists
This is exactly why I founded H₂HUBB. Unlike a manufacturer or distributor, H2HUBB doesn’t own or sell H₂ devices directly. We don’t have a warehouse full of machines we need to move, and that gives us the freedom to evaluate products on their actual performance.
What we do is simple, powerful and needed: we test, verify, and approve devices against independent internal performance standards. That means when you see an H2HUBB-approved product, you know it’s been examined first by us for safety, for therapeutic potential, and for legitimacy. You don’t have to take a company’s word for it—you can lean on trusted and credible third-party testing.
Now, let me be transparent here: no one, not even H2HUBB, is without bias. My bias is clear—I believe in hydrogen therapy. I’m convinced, from years of testing and from the scientific medical data itself, that hydrogen can benefit human health. So yes, my “bias lens” is that I want to see hydrogen succeed, and I want people using it safely and effectively through tested products we have examined in house. But beyond that, we have no attachment to any one product. If a device doesn’t meet the standard, we don’t recommend it. That’s the difference between a company protecting its sales and an independent third party protecting the consumer.
This overview guide exists for that reason—to lay out the strengths, limitations, and misconceptions of each major type of hydrogen inhalation device so you can see clearly where each one fits. There isn’t a single “best device” for everyone. Each type has contexts where it works well and contexts where it might not. My goal is to give you the framework so you can see through the marketing noise and make a confident, informed decision.
Fundamentals: What Is Hydrogen Inhalation?
Before we dig into specific devices, let’s make sure we’re on the same page about what hydrogen inhalation actually is.
At its core, hydrogen inhalation is the process of breathing in molecular hydrogen gas at an inhaled concentration high enough to have therapeutic effects. Human clinical studies typically use concentrations in the range of 1–4% hydrogen in air [7], though a growing body of preclinical and clinical human research goes beyond that [8][9][10].
To understand what that really means, think about your breathing. At rest, most adults breathe about 5–8 liters of air per minute [11][12][13]. If we take the middle of that range—say 6 liters per minute—then 1–4% of that air would need to be hydrogen gas in order to deliver a therapeutic dose. That translates into somewhere between 60 and 240 milliliters of hydrogen per minute actually entering your lungs. Now, those numbers will need to be higher with nasal cannulas as there are losses but this gives you an idea and the starting point: therapeutic inhalation means consistently breathing hydrogen in that ballpark or higher.
This matters because inhalation is arguably the most efficient way to get hydrogen gas into the body [14][15]. Unlike hydrogen water, which has to pass through the digestive tract, or hydrogen saline, which requires injection, inhaled hydrogen goes straight to the lungs, into the bloodstream, and from there it’s distributed systemically [16]. It’s fast, it’s efficient, and it’s measurable [17]. That’s why inhalation has become such a focus in both the scientific literature and the therapeutic market.
The Takeaways
- Hydrogen inhalation = breathing molecular hydrogen at therapeutic concentrations (usually 1–4% or higher).
- Average adult ventilation is 5–8 L/min. At 1–4% inhaled H₂ concentration, that translates to roughly 50–320 mL of hydrogen per minute. However, when delivered through a nasal cannula, you have to account for exhalation losses, so the device may need to supply closer to 150–960 mL/min to achieve the same effective dose.
- Inhalation provides the most systemic exposure compared to water.
- Clinical studies have often used tanks with precisely controlled gas mixtures; consumer devices attempt to replicate this using electrolysis.
How Hydrogen Inhalation Devices Work
Now that we’ve established a foundation of what hydrogen inhalation actually is, let’s talk about how the devices on the market try to deliver it. Because here’s the truth: when you see a hydrogen inhalation unit online—whether it’s marketed as pure hydrogen, Brown’s Gas, or H₂ mixed with air—they’re all attempting to do the same basic thing: generate hydrogen gas from water and then deliver it through your lungs to your body in a usable, therapeutic way. The way they get there, however, can look very different.
Most devices fall into three main categories:
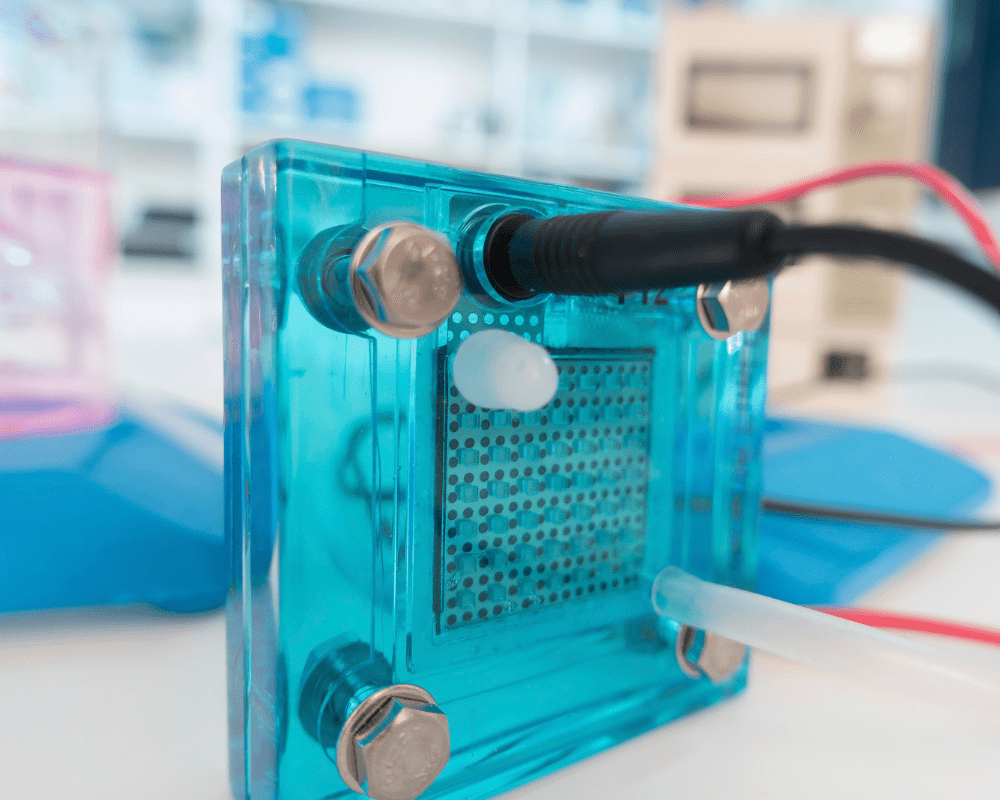
Pure Hydrogen (PEM/SPE) Devices
Pure hydrogen inhalation devices utilize proton exchange membrane (PEM/SPE) technology to decompose water molecules into hydrogen gas and oxygen gas. Unlike alkaline electrolysis systems, they only require distilled water—no added mineral ions or chemical agents. On the cathode side of the electrolysis assembly, the device generates hydrogen gas with purity levels greater than 99.9%. On the anode side of the electrolysis assembly, pure oxygen is produced.
Each manufacturer may handle that oxygen differently: some devices off-gas or vent it into the air as waste, while others have dedicated oxygen ports on the system for re-routing it into tubing that allows both hydrogen gas and oxygen gas to be inhaled together. This is optional oxyhydrogen therapy via a PEM/SPE device, but at the core these devices are designed to deliver pure molecular hydrogen.
Strengths
PEM/SPE systems are generally considered the gold standard for purity among the available H₂ inhalation units on the market currently. The gases (H₂/O₂) are completely separated in the electrolysis cell produced from distilled water, which means the hydrogen gas delivered to the user is clean and free from electrolyte contamination.
Regarding a safety perspective, pure hydrogen devices also have some advantages in comparison to some other models. Because hydrogen and oxygen remain completely isolated within the device housing until they exit at their respective user ports, no combustible gas mixture ever travels through the internal system. The only place where hydrogen gas mixes with oxygen is at the tip of the nasal cannula when it enters the atmosphere, right where it enters the nose [18]. That means a combustion cannot travel backward into the device if pure H₂ is only applied to the nasal cannula tubing, as 99% hydrogen gas is not combustible [19].
It’s also important to remember the physical nature of molecular hydrogen:
- It’s the smallest and lightest gas [20].
- It diffuses extremely quickly in air [21][22].
- And during breathing, inhaled gas mixes immediately with the large volume of air moving in and out of the lungs [23].
So while the device may output nearly 100% hydrogen, by the time it mixes with atmospheric air in the nasal cavity, concentrations drop rapidly into the therapeutic range and quickly below combustible levels depending on the delivered flow rate of hydrogen gas. This explains why, when engineered properly, PEM/SPE devices can carry a high safety profile.
Lastly, maintenance is simple: refill with distilled water when the reservoir runs low, clean or replace nasal cannulas, and drain/refill the system about once a month are the main essentials.
Limitations
No system is without limitations. One limitation is that PEM/SPE cells do have a lifespan—typically 4,000–10,000 hours for most well engineered pure H₂ systems before their output begins to decline. Replacing the cell requires service from the distributor or manufacturer, which can add cost over time.
Another limitation comes from the delivery method itself. Pure H₂ devices have to use nasal cannulas or simple O2 masks to obtain O2 with the inhaled breath, and this introduces variability. The majority of the hydrogen gas loss is during exhalation. Breathing patterns among humans vary. With these systems hydrogen gas production and flow is always going during non-inhalation phases (except for Pure H₂ devices that use pulse mode). This means the actual inhaled concentration may fluctuate, and if so, so does blood and plasma H₂ levels.
Does this variability undermine the therapy? Currently we do not have enough data that suggest it does. We do have clinical studies that show excellent results even with nasal cannula delivery with pure H₂ devices. For example, a 2022 rehabilitation study on COVID-19 patients demonstrated that inhaling 300 mL/min of pure hydrogen (>99.99%) via nasal cannula led to significant clinical benefits [24]. This is promising because it provides evidence that this inherent variability may not be a barrier to therapeutic use.

“Pure H₂ Inhalation Causes Hypoxia”
One of the most common misconceptions about pure hydrogen inhalation is that breathing H₂ through a cannula will “push out” oxygen and inevitably cause hypoxia. At first glance, the idea makes sense—if you’re adding hydrogen gas, you must be reducing oxygen gas, right? But when you actually break down the physiology and the math, the claim doesn’t hold up under real-world conditions.
The physiology:
- Room air contains ~21% O₂ (20.95%) [25].
- With each breath, we only uptake approx 5% and exhaled air still contains 16% O₂. In other words, we inhale far more oxygen than we use [26].
- At rest, the average adult uptakes ~250–300 mL of O₂ per minute (based on a tidal volume of 500-600 mL and ~12 breaths per minute) [27][28].
- Mild hypoxia symptoms generally appear when inspired oxygen drops below 16%. Serious hypoxia or loss of consciousness doesn’t occur until inspired oxygen is down around 10–11% [30][31][32][33].
The math with pure H₂ delivery:
Let’s consider a typical scenario: 6 L/min of ventilation (inhalation and exhalation), with 2 L/min of pure H₂ delivered through a cannula, and assume 100% capture of that hydrogen gas during the 20 seconds of inhalation each minute.
- Baseline inspired oxygen = 20.95%
- With 2 L/min H₂ → inspired oxygen ≈ 18–19%
- Even with 3.4 L/min H₂ captured at 100% efficiency → inspired oxygen ≈ 17%
- Raise ventilation to 7.5 L/min (large healthy adult male) → inspired oxygen stays closer to 18–19%
This means that under common conditions with nasal cannulas, oxygen rarely drops into the symptomatic hypoxic range for healthy adults.
Simple step-by-step example
Assumptions:
- Minute ventilation (VE) = 6 L/min (6,000 mL/min)
- You inhale for 20 seconds each minute (the other ~40 s you’re exhaling)
- Room air O₂ = 20.95%
- Device H₂ flow fate (F) = 2.0 L/min of pure H₂ (so 0% O₂ in the delivered gas)
Step-by-step
How much air you inhale per second (during the 20 s of inhalation):
6000 mL/min ÷ 20 s = 300 mL/s
How much hydrogen the device sends per second:
2,000 mL/min ÷ 60 = 33.33 mL/s
Room air replaced by that hydrogen each second:
300 − 33.33 = 266.67 mL/s of room air
O₂ you’re now getting from room air each second:
266.67 × 0.2095 = 55.86 mL O₂/s
O₂ per minute (only inhale 20 s each minute):
55.86 × 20 = 1,117.34 mL O₂/min
Convert that to an oxygen percentage of your total breathing (inspired oxygen):
Divide by total minute ventilation (6,000 mL/min):
1,117.34 ÷ 6,000 = 0.1862 ⇒ 18.62% O₂
What changed?
- Baseline oxygen (room air): 20.95%
- With 2 L/min pure H₂: ~18.62%
- Drop: about 2.32 percentage (in the 18–19% range)
This particular case involved some of the highest hydrogen flow rates possible, whereas the vast majority of devices on the market deliver far less—typically under 2 L/min.
The difference between hypoxemia and hypoxia:
For true tissue hypoxia to occur, you’d first need to see hypoxemia (low blood oxygen). That typically requires oxygen uptake to fall below ~200–300 mL/min. Achieving that with nasal-cannula pure H₂ would demand extremely high displacement H₂ flow rates that 95% of current devices simply don’t produce.
Context matters: nasal cannulas vs sealed systems
It’s important to be clear that these calculations specifically apply to nasal cannula use. With a sealed mask or rebreather system, localized effects could differ depending on the gas mixture supplied, and mild hypoxia, hyperoxia, or hypoxemia localized to the lungs could theoretically occur depending on system design and inspired oxygen delivery. But for typical home use with cannulas, the rapid dilution of hydrogen gas into ambient air during non-inhalation phases, combined with the body’s oxygen reserves, means that “pure H₂ automatically causes hypoxia” is not supported by physiology, math, or clinical research.
Important note: The Science Behind FiH₂ and H₂ Dose Estimation
It’s important to give acknowledgment to the medical researchers whose work helped inform H2HUBB’s understanding of how to calculate or form estimates for inhaled hydrogen concentrations. One foundational study for us was Sano et al. (2020), “Low-Flow Nasal Cannula Hydrogen Therapy” (Journal of Clinical Medicine Research, 12:674–681), providing some insights into how hydrogen flow rate relates to tidal inhaled H₂ concentration and subsequent blood H₂ levels. This research gave us a valuable benchmark for understanding these physiological relationships five years ago.
Even more importantly, I want to recognize Dr. Tyler W. LeBaron and the Molecular Hydrogen Institute (MHI) for their foundational work on calculating both inspired oxygen (FiO₂) and inspired hydrogen (FiH₂) concentrations from hydrogen inhalation devices. Their contributions have been instrumental in shaping how we analyze and interpret hydrogen delivery across different device types.
H2HUBB could not have formed these estimations methods and examples shared in this article without the prior work of Dr. LeBaron, MHI, and other pioneering researchers in this field. If you want to take a deeper dive into the science behind the fraction of inspired hydrogen (FiH₂), gas exchange dynamics, and other technical aspects of hydrogen inhalation, I highly recommend enrolling in the MHI Level 1 and Level 2 Certification Courses. Through H2HUBB, you can receive 10% off your enrollment.
Our simplified estimation models in this article serve as a practical demonstration to help clarify and debunk misconceptions surrounding pure hydrogen inhalation and hypoxia—but for a more comprehensive understanding, MHI’s coursework provides essential education on hydrogen inhalation physiology.
The bottom line:
- Pure H₂ does displace a small fraction of oxygen—just like any other gas supplied to the nasal cavity would—but not nearly enough to cause tissue hypoxia in normal users for most systems.
- Even at the extreme worst-case of 4.0 L/min, inspired oxygen drops to 16.7% at 6.5L/min VE — still above the point where true hypoxemia and tissue hypoxia occur.
- The “hypoxia” claim is really a misinterpretation—it sounds logical, but it ignores how much oxygen reserve the human body actually has and how much hydrogen gas you’d really need to cause a problem.
There are certain cases—such as pediatric patients, people with advanced pulmonary disease, or extreme high-flow setups—that do require extra caution and possibly medical oversight. But for typical home use with well-engineered moderate-flow rate pure H₂ devices, the risk of hypoxia is low supported by physiology, math, or published research.
“Why “More Oxygen” Isn’t Always Better”
Some companies use this argument to promote oxyhydrogen devices, claiming that mixing in oxygen makes their units safer and more therapeutic. But supplemental oxygen is not inherently “better.”
In fact, clinical studies show that breathing excess oxygen—even briefly—can increase localized oxidative stress markers [34]. Oxygen is a drug, and like any drug, dose and context matter. While there are medical situations where added O₂ is appropriate, it’s not a blanket reason to dismiss pure hydrogen delivery.
A Few Remarks on the Oxyhydrogen Reviews
There’s a review papers that often gets shared to support these anti–pure hydrogen claims.
But these papers has some issues:
- Some use the 19.5% oxygen threshold—a workplace safety cutoff for confined spaces from OSHA—as if it were a medical threshold for hypoxia. That’s misleading [35].The publication reads more like a marketing poly with conflict of interest cited in the paper, than well rounded science.
- Some label 10% hydrogen as a “critical explosive threshold,” which oversimplifies combustion science and conflates fast burns (“pops” or small non-harmful deflagration) with true detonation [36].
- Some generalize the safety features of one company’s design to an entire category, ignoring the diversity of engineering approaches.
In short, some of these reviews read more like a marketing piece than neutral science. While it does highlight real risks—hydrogen is flammable, and safety engineering matters—it should not be used to categorically dismiss pure hydrogen cannula therapy.
Safety
Just like hyperbaric oxygen therapy, hydrogen inhalation safety depends on the context, system design, and user protocol. Well-engineered pure hydrogen devices and other types of H2 inhalation system typically include:
- Enclosed plumbing system for the gas-path that keeps hydrogen non-combustible until mixing occurs at the cannula.
- Moisture stages (humidifiers) that reduce static and improve gas purity.
- One-way valves and gas-water separators to stop backflow and improve purity.
- Internal fans or vents to disperse leaks if occurred.
- Auto-shutoff systems for overheating, voltage surges, leaks, or gas blockages.
For users, the basics apply: use the device in a ventilated room, keep away from flames and sparks, handle tubing properly, and follow the manufacturer’s instructions.
The Takeaways
Pure hydrogen inhalation devices are safe, clean, and effective when properly engineered and used. The idea that they inherently cause hypoxia is an overgeneralization.
The reality is:
- With standard flow rates and nasal cannulas, inspired oxygen typically stays above concern thresholds in healthy users for most devices.
- Oxyhydrogen is not automatically safer just because it includes oxygen—context matters.
- Device safety is about engineering and design, not just which gas is being delivered.
At H2HUBB, this is why we evaluate both performance and safety design. We don’t endorse units simply because they produce hydrogen gas—we approve them when they meet independent internal standards for therapeutic delivery and user safety.
Oxyhydrogen (Brown’s Gas) Devices (No Membrane)
I want to preface this section with an important notice, because out of the three types of hydrogen inhalation units in this article, this one carries the most controversy and attracts some of the strongest, almost dogmatic support. A lot has been said online about Oxyhydrogen or Brown’s Gas (BG) generated from no-membrane electrolyzers — claims about its supposedly greater therapeutic potential, its unique gas mixture composition, its so-called “exotic gaseous plasma state” produced alongside H₂/O₂, and the list goes on.
At H2HUBB, our commitment has always been, and will continue to be, to cite and educate from peer-reviewed, published scientific research — a growing body of data that aligns with the established principles of chemistry, biology, and physics. This approach can sometimes feel limiting to those drawn to cutting-edge theories, fringe science, or early-stage innovations that haven’t yet passed through the rigorous process of validation. But that limitation is also a safeguard. It protects us — and the public — from pseudoscience, conjecture, and outright false claims.
That does not mean peer-review research is infallible. Like all human effort, it is subject to error. But as of today, empirical research remains the best tool we have for making objective claims about safety and therapeutic potential.
Therefore, this section will address oxyhydrogen devices based on established peer-reviewed medical research and the foundational laws of physical chemistry. I’ve stated this publicly and privately, including to some of the most vocal BG proponents in the world, and they know exactly where H2HUBB stands on the subject. With that said, let’s begin.
Oxyhydrogen devices operate using alkaline electrolysis, which requires adding an electrolyte such as potassium hydroxide (KOH) or sodium hydroxide (NaOH) to distilled water to create the electrolysis solution. This solution is supplied to an electrolysis cell that has no membrane separating the cathode and anode. As a result, the process does not divide the gases but instead generates hydrogen and oxygen together in a natural 2:1 ratio — approximately 66.67% hydrogen and 33.34% oxygen [37].
This mixture is what’s commonly referred to as oxyhydrogen or Brown’s Gas (BG). Advocates of BG argue that the combination of hydrogen and oxygen offers unique therapeutic benefits and may enhance the overall effect. And there’s some interesting theories—but here’s the important thing: because BG is a flammable and detonable mixture, it comes with different safety considerations than pure hydrogen devices [38].
Strengths
One of the genuine strengths of certain oxyhydrogen units is cost-effectiveness. Some models are capable of delivering relatively high flow rates of hydrogen gas at a lower price point compared to PEM systems. For individuals looking to achieve therapeutic hydrogen output without making a major financial investment, this can be an appealing option.
Another strength is that some of the companies producing oxyhydrogen (Brown’s gas, no-membrane) devices have been in the industry for many years and back their products with strong warranties. This kind of long-standing presence and after-sale support can be attractive to consumers who are weighing both performance and reliability when investing in a device.
A third potential strength is the additional oxygen these systems provide. Because oxyhydrogen is produced in its natural ratio of approximately 66% hydrogen and 33% oxygen, the mixture contains about 12% more oxygen than the 21% oxygen we normally breathe. Now, this needs to be framed carefully. As I’ve explained before, oxyhydrogen is inherently a combustible (flammable and detonable) mixture, so the extra oxygen is not automatically an advantage that makes these devices superior. Context matters. I’ll unpack that more in the misconceptions section. That said, in certain situations—such as COPD, COVID-19 rehabilitation, or patients with compromised lungs or heart conditions—the additional oxygen may offer some benefit, especially under medical supervision or in a controlled clinical environment.
Limitations
When it comes to oxyhydrogen (BG) devices, this is where things really need to be weighed carefully. Unlike pure H₂ systems, the gas mixture inside oxyhydrogen (BG) units is combustible and detonable not only at the cannula tip, but throughout the entire device— from the electrolysis cell, through the tubing, and into the delivery interface [39]. That means the entire system must be engineered with layered safety components in place, such as flame arrestors, humidifiers, one-way valves, and proper venting designs [40]. Without those redundancies, the risks are far greater than with PEM/SPE-based pure H₂ systems.
Another area of concern is electrolyte handling. These devices require an alkaline electrolyte (usually sodium hydroxide, NaOH/lye, or potassium hydroxide, KOH) to drive the electrolysis process. While effective for conductivity, these are caustic chemicals, meaning mixing, storage, and replacement must be handled correctly. Improper handling risks skin or eye burns, spills, or long-term corrosion of the device. Users need to be far more attentive compared to the near “plug-and-play” nature of PEM/SPE systems.
There is also the matter of electrode wear and solution degradation. Alkaline environments are hard on electrode plates. Depending on the electrode material and use patterns, metals like chromium and iron can leach into the solution, and the plates themselves can wear down more quickly than PEM membranes due to the harsh environment. This means a higher maintenance burden, greater potential for contamination, and more frequent need for servicing or replacement parts.
On top of that, if anything but true distilled or double-distilled water is used, unwanted byproducts can form. Chloride ions in tap or mineral water, for example, are preferentially oxidized at the anode, producing chlorine gas (Cl₂) and other chlorine species. In some cases, trace ozone (O₃) or hydrogen peroxide (H₂O₂) can also form. These are gases you don’t want delivered to the lungs. For this reason, strict adherence to water purity is non-negotiable.
And this is exactly why H2HUBB has conducted its own tests on one of the oxyhydrogen (BG) units we have evaluated. As of today, there is no established IHSA standard for oxyhydrogen (BG) purity, and manufacturers are not typically providing laboratory gas analyses. That means we have to evaluate performance ourselves before considering any recommendation. Our durational TDS and pH testing showed that after 10–15 hours of use, the first-stage humidifier water climbed to just over 1000 TDS — equivalent to 0.9% saline solution. The secondary bubbler water also increased slightly in TDS (24 ppm, up from 0 ppm with distilled water), with a minor pH rise. This confirmed that the humidifier water was actively scrubbing the NaOH mist from the gas stream.
Now, these results should not be taken as representative of all oxyhydrogen (BG) units, since every system will differ in gas purity and scrubbing efficiency depending on its design. However, what these tests do demonstrate in principle is that distilled water in humidifiers can effectively clean the gas — and that properly designed humidifier systems are absolutely essential for the safe operation of oxyhydrogen (BG) devices. Based on this evaluation, H2HUBB recommends changing humidifier water regularly to ensure the gas remains free of contaminants.
Finally, oxyhydrogen (BG) units come with a heavier maintenance burden overall. Between electrolyte replacement, plate monitoring, and ensuring that flame arrestors, check valves, and traps are functioning properly, these systems demand ongoing attention to remain safe. They can be cost-effective and even beneficial in certain cases, but that lower upfront price comes with higher complexity and user responsibility.

“Brown’s Gas is better than pure H₂.”
This is one of the most common claims I hear, but it’s not supported by head-to-head human clinical trials. To date, there is no published peer-reviewed evidence showing that Brown’s Gas (oxyhydrogen) is inherently superior to pure hydrogen.
Here are a few of the scientific studies we have on record: a handful of comparison studies across different administration methods and concentrations.
- H₂ water vs. H₂ inhalation (animal model): [41]
- High-concentration H₂ inhalation (67%) vs. low (4%) (animal model): [42]
- 1.2 mg/L H₂ water vs. 4% H₂ inhalation (animal model): [43]
- High vs. low concentration H₂ water (animal model): [44]
- H₂ inhalation 1%, 2%, 3% (3% greater effect in SCI model): [45]
- H₂ inhalation vs. H₂ saline (animal model): [46]
- 42% H₂ vs. 3% H₂ inhalation (animal model): [47]
- High vs. low concentration H₂ water (animal model): [48]
- Blood H₂ levels, 3% vs. 4% inhalation (human study): [49]
That’s a short list, and importantly, none of these directly compare oxyhydrogen to pure H₂ in humans. I’m not aware of any active H2 researcher claiming BG is categorically superior on the basis of supplying extra oxygen or producing so-called ExW (electrically expanded water).
Here’s why this matters. People want simple, definitive answers to complex, dynamic biological processes. It’s easy to give a speculative explanation and make it sound like fact—especially if you’re trying to sell devices. But when you look at the actual data, the consistent theme is dose-dependence. Higher inhaled hydrogen concentrations or higher dissolved H₂ levels in water often outperform lower ones across different models.
A few examples:
- In traumatic brain injury, 42% H₂ outperformed 3% H₂ [50].
- In spinal cord injury, 3% H₂ outperformed 1% and 2% [51].
- In sepsis, 67% H₂ outperformed 2% [52].
- In a neural survival model, 75% H₂ outperformed 50% and 65% [53].
At the same time, not every result is identical or linear. One study in NAFLD showed both 4% and 67% H₂ inhalation had benefits, with each outperforming the other in different ways [54]. That suggests high- and low-concentration H₂ may activate distinct mechanisms of action depending on the disease model. In the future, we may well discover optimal inhaled doses for different conditions—1.3% for one, 4% for another, considerably higher for yet another.
But here’s the critical point: these benefits are coming from hydrogen gas itself and how much is being delivered—not because oxyhydrogen has a unique therapeutic property as a mixed gas.
Many oxyhydrogen studies simply use very high flow rates—often around 3,000 mL/min of mixed gas (H₂/O₂), which equates to ~2,000 mL/min of hydrogen. That’s a substantial therapeutic dose. If you took a pure hydrogen system and delivered the same inhaled H₂ concentration to the lungs, you’d expect comparable results. The “magic” isn’t in the Brown’s Gas label—it’s in the dose of hydrogen reaching the body.
“The extra oxygen makes oxyhydrogen (BG) more therapeutic.”
Another common argument is that oxyhydrogen (Brown’s Gas) is superior because it contains ~33% oxygen compared to the ~21% oxygen in room air—a relative increase of about 12%. On paper, this sounds like a meaningful improvement.
But once you actually deliver that gas through a nasal cannula, the reality looks very different. Dilution and losses into ambient air during non-inhalation phases dramatically reduce the “oxygen bump.” For example, at a flow rate of ~1,000 mL/min of BG, the inspired oxygen rises less than 1 percentage—from about 21.0% to ~21.8%. That is not nearly enough to meaningfully change blood oxygen saturation in a healthy person, because hemoglobin is already ~95–98% saturated under baseline conditions [55][56]. In other words, our red blood cells are carrying almost as much oxygen as they possibly can.
And here’s the key point: oxygen exposure is not the same as oxygen uptake. Simply raising the percentage of O₂ in the inhaled breath does not guarantee more oxygen enters the blood. Uptake is governed by partial pressure (pO₂) and hemoglobin’s affinity for oxygen, which is already near full capacity. Unless you meaningfully raise alveolar pO₂—as in hyperbaric oxygen therapy—you cannot force hemoglobin to carry significantly more oxygen [57][58].
This is why physiology matters. The Bohr effect shows how shifts in CO₂ and pH regulate oxygen release to tissues, while the Haldane effect explains how deoxygenated hemoglobin more effectively carries CO₂ back to the lungs [59][60]. Together, these mechanisms tightly control gas exchange. Adding extra oxygen to the inhaled breath—especially in small amounts like 12% above room air—doesn’t override these regulatory systems [61][62].
It’s also important to be precise: my comments here apply specifically to nasal cannula use. With a sealed mask or rebreather setup, the situation may differ, as localized mild hyperoxia in the lungs could occur. That said, under typical home use with nasal cannulas, the added oxygen is diluted or lost during non-inhalation phases before it reaches the alveoli, and hemoglobin saturation prevents it from meaningfully changing systemic oxygen delivery.
So while BG does provide more O₂ on paper, in practice that additional oxygen is minimal when used with a cannula. And we must weigh the trade-off: combining hydrogen and oxygen in one gas stream inherently creates a combustible, detonable, mixture. That added risk is real, whereas the therapeutic gain from “extra oxygen” is limited.
“ExW (Electrically Expanded Water) proves BG is superior.”
The idea of Electrically Expanded Water (ExW) is often promoted in oxyhydrogen circles. It suggests that these devices produce a unique plasma-like water state that carries additional therapeutic benefits. It’s an intriguing hypothesis, but here’s the reality: there is no peer-reviewed clinical evidence we are aware of demonstrating that ExW exists and that it provides distinct therapeutic effects. Right now, ExW is speculative and has not been substantiated by replicated peer-reviewed empirical evidence.
It’s possible that ExW—or something like it—exists and may carry potential benefits. But without adequate research to validate or confirm these claims, we simply don’t know. And it’s not just the potential benefits that need to be considered. Any new discovery also comes with the possibility of harms. This is true for hydrogen therapy as well. That’s why many hydrogen studies don’t just report benefits—they also evaluate safety profiles and possible adverse effects.
What’s remarkable is that with more than 3,000 scientific publications and upward of 200 different disease models studied—including human phase I, II, and even phase III clinical trials underway or already conducted—molecular hydrogen (H₂) has consistently demonstrated therapeutic effects with little to no documented adverse outcomes [63][64][65][66][67]. Still, we must acknowledge that in the future there may be rare disease states where H₂ is contraindicated because its research is still in its infancy.
The same logic applies to ExW. At present, we have minimal data suggesting it exists, and virtually no information about its possible beneficial or negative effects—short- or long-term. Brown’s Gas proponents may very well be onto something, but in my opinion it’s far too early to jump on the bandwagon. It is safer and more responsible to focus on what the evidence clearly supports: hydrogen therapy itself, which is already on the frontier of medical gas research with a growing body of data demonstrating its therapeutic value.
Safety
Because oxyhydrogen or Brown’s Gas (BG) devices are flammable and detonable throughout the entire line, safety engineering is critical.
Well-built systems use:
- Flame arrestors close to the cannula.
- One-way valves to prevent backflow.
- Bubblers and separators to stop electrolyte carryover.
- Sealed internal plumbing and venting fans.
- Auto-shutoff systems for leaks, overheating, or blockages.
And for users: always use distilled/double-distilled water, handle NaOH carefully, use the device in a well ventilated the room, and keep away from open flames or sparks. These are not “plug-and-play” machines—you have to respect the chemistry and maintain them properly.
The Takeaways
Oxyhydrogen devices can be therapeutic, but they are not automatically superior to pure hydrogen units.
- Their main strengths are cost (in some models) and contextual oxygen support.
- Their main limitations are higher safety concerns, maintenance, caustic electrolyte handling, and electrode degradation.
- The misconceptions—that BG is inherently better, that the oxygen increase is game-changing, or that ExW is proven—don’t hold up under scrutiny.
- The impressive results in some BG studies may often come down to dose: higher hydrogen concentrations and higher flow rates may produce stronger responses, no matter the system.
If you choose BG, do it because you’ve found a well-engineered unit and you’re comfortable with the safety and maintenance requirements—not because of marketing claims. For most people, especially those new to H₂ therapy, starting with a pure hydrogen PEM/SPE unit makes more sense.
Hydrogen Mixed with Air Devices
Hydrogen mixed-with-air systems are the third major category of inhalation devices. Instead of delivering pure hydrogen or a hydrogen/oxygen mixture (Brown’s Gas), these units take a different approach: they generate hydrogen gas via PEM electrolysis, then mix it directly with ambient air to non-combustible levels before you ever inhale it.
One example is the Inhale H₂ device that we at H₂HUBB tested and approved.
This unit uses a PEM/SPE electrolytic cell to make >99.9% pure hydrogen, then mixes that with a steady 12 L/min airflow to deliver a controlled 1–4% hydrogen concentration. Importantly, it uses a specialized sealed dual-valve rebreather mask that guarantees each inhaled breath maintains that set concentration, without the fluctuations you get from nasal cannulas [68]. This unit will soon become the gold standard for safety regarding hydrogen inhalation systems due to its design and engineering.
But not all mixed-air devices work this way. Some, like the MHG-2000α [69], have used nasal cannulas for delivery. That’s a problem, because the hydrogen-air flow may not be enough to replace the full tidal volume of a normal breath. With nasal cannulas, additional dilution occurs as the gas mixes with surrounding room air, making it difficult to guarantee the user is actually receiving a therapeutic dose. Therefore, the device is already premixing and diluting the hydrogen gas, and with nasal cannulas or simple O₂ masks that are not sealed, the gas is diluted again at the nasal cavity — further decreasing the chances of providing therapeutic levels of hydrogen.
This is why design matters so much. Some of these units have not been verified by H2HUBB to ensure the hydrogen gas production and final hydrogen-with-air mixture is sufficient to deliver the intended concentration, or that enough of it actually reaches the user to ensure therapeutic potential.
Strengths
The greatest strength of hydrogen mixed-with-air systems is their exceptional safety profile. Because these units premix pure hydrogen gas with large volumes of air inside the device, the gas is already diluted to non-combustible levels before it ever reaches the user. NASA lists the lower flammability limit of hydrogen gas in air at about 4.6% and the detonability threshold beginning around 18.3% [70], and these values are well supported by other published sources [71][72]. These systems are engineered to remain well below that threshold. In practical terms, this makes them essentially non-flammable and non-detonable under normal operation, giving them the highest margin of safety of any hydrogen inhalation technology.
Another key advantage is the ability to achieve controlled, consistent dosing when paired with specialized sealed hydrogen masks. Unlike nasal cannulas—which are prone to significant gas losses and fluctuations—these systems can maintain a stable fraction of inspired hydrogen breath after breath. That stability is not just a technical detail; it’s a major benefit in clinical and research settings where reproducibility and precision are critical.
Finally, their design makes them particularly well suited for clinics, hospitals, research labs, and elderly care facilities. Because they operate for long durations, remain safely below explosive thresholds, and deliver hydrogen in the therapeutic range of 1–4%, they provide both reliability and peace of mind. For institutions that require both safety and efficacy, hydrogen mixed-with-air devices stand out as a compelling option.
Limitations
One of the biggest limitations of mixed-air systems is their built-in ceiling. Most of these units max out at around 4% inhaled hydrogen. Now, that’s not inherently bad. There’s a difference between something being bad and something being a limitation. The 1–4% range has been intentionally used in much of the human and animal literature, largely to avoid flammability risks in clinical settings, and these levels have demonstrated clear therapeutic effects [73][74][75].
But when you consider the broader body of human clinical research, you’ll notice a wide spread of administered hydrogen doses—many well above 4%. Concentrations in the 5–12% range have been used with impressive results [76][77][78][79][80][81][82], from studies conducted in Japan and China. This raises an important point: while mixed-air devices do deliver clinically relevant concentrations, they may not always align with the higher-dose administrations seen in some impactful studies.
The research trend continues to highlight a dose-dependent response—where higher inhaled concentrations or hydrogen water concentrations, and therefore higher subsequent blood and cellular hydrogen levels, tend to outperform lower ones [83][84][85][86].
“A dose–response effect of hydrogen is observed in drinking hydrogen-rich water [94, 97]. A similar dose–response effect is also observed in inhaled hydrogen gas [1, 17, 98].”[87]
This suggests that while mixed-air systems excel in safety, they may also be therapeutically capped compared to higher flow rate pure hydrogen or oxyhydrogen units, particularly for certain disease models where higher dosing was used in the study. We cannot simply assume that inhaling hydrogen at 4% will produce the same effects as 8%; in fact, current studies suggest the opposite may be true, though further research may be needed to fully confirm this emerging trend.
Another key limitation comes from how some mixed-air devices are engineered. Units that rely on nasal cannulas with low H2 flow rates often fall short of delivering what they claim. The issue is straightforward: if the device’s hydrogen-air output does not equal or exceed a normal tidal volume (roughly 400–600 mL per breath for an adult), the user has to draw in additional room air to complete each breath. That extra atmospheric air dilutes the hydrogen mixture even further.
On paper, a manufacturer may state their system delivers, say, 6–7% H₂ in the gas stream. But once that gas is routed through a nasal cannula and combined with room air to fill the rest of the tidal volume, the actual inhaled H₂ concentration reaching the lungs can fall significantly below the advertised value. For example, as we’ve shown earlier, even when a device premixes hydrogen with air, the gas may be diluted a second time in the nasal cavity, lowering the effective inhaled H₂ concentration even further.
This means consumers may believe they are inhaling a precise concentration, but with cannula-based systems, that concentration may not be guaranteed in real-world use. The end result is that the therapeutic dose of hydrogen delivered to the body could be much lower than expected. This is why design matters so much—and why specialized sealed masks or rebreather-style delivery systems are a far superior option when consistency and accuracy are important to ensure therapeutic value.
Finally, mixed-air systems are less versatile compared to pure hydrogen or oxyhydrogen technologies. By design, they are single-user devices and capped at lower hydrogen outputs in order to maintain their exceptional safety profile. While this makes them very safe, it also means they operate within a narrower therapeutic window.
In contrast, several pure H₂ and oxyhydrogen units come standard with enough hydrogen output to supply two users simultaneously. That kind of capacity simply isn’t possible with the current mixed-air systems on the market. The single-user, lower-concentration design is a real strength when it comes to minimizing risk, but it also comes with trade-offs: these devices may not achieve the higher concentrations that certain therapeutic contexts or research protocols are being explored.
Unlike pure hydrogen or oxyhydrogen systems, which can be scaled to deliver higher flows and concentrations, mixed-air units remain intentionally conservative. For many users and clinical conditions, that’s more than enough. But for others, the built-in therapeutic ceiling and lack of multi-user versatility could become limiting factors.
“1–4% is all you’ll ever need.”
It’s true that 1–4% hydrogen inhalation has demonstrated therapeutic effects in multiple human studies. That’s why many mixed-air devices are designed around this range—it’s safe, non-flammable, and supported by data [88][89]. But as we’ve already covered throughout this article, the broader body of research points to a dose-dependent response. In both animal and human studies, higher hydrogen concentrations often outperform lower ones, depending on the disease state and context.
For example, many human cancer trials have administered hydrogen at flow rates around 2,000 mL/min. Given typical ventilation, that likely results in an inhaled H2 concentration of 8–12% [90][91][92]. Those concentrations are well above what mixed-air systems can deliver. This doesn’t mean mixed-air devices are ineffective—they certainly provide therapeutic value. What it does mean is that they may not always reach the same levels seen in clinical articles exploring higher-dose hydrogen inhalation.
Right now, we don’t have a consensus on hydrogen dosing, nor enough consistent data across disease states to draw definitive conclusions. The best approach at this stage is to align as closely as possible with the dosing protocols used in medical studies that have already shown positive results. That gives us the strongest foundation for responsible practice while more dosing research continues to emerge.
Ultimately, this is why each device type has its place. Mixed-air systems may be ideal for safety-focused users, clinics, or those who only need moderate doses, while higher-output pure hydrogen or oxyhydrogen devices may be better suited for certain disease states or contexts where higher inhaled H₂ concentrations has shown benefit. In other words, it’s not about one device being universally better—it’s about matching the tool to the therapeutic need.
“If it’s not H₂ + air, it’s automatically unsafe and harmful.”
This one comes up a lot in the industry. Some argue that the only safe way to inhale hydrogen is by premixing it with air before delivery, and they’ll often point to case reports or incident write-ups to back the claim. One of the most recent examples is a 2024 case report by Tsuchikane et al. in Acute Medicine & Surgery, titled “A case of lung injury due to a hydrogen explosion caused by the simultaneous use of two home folk remedies devices.” [93]
On the surface, the story sounds alarming, which it should. A 62-year-old woman, recovering from breast cancer surgery, was inhaling hydrogen via nasal cannula while also applying an EM-wave/heat device near her chest. She reported hearing an “explosive” sound, smelled burning, and later developed hemoptysis (coughing blood). CT scans showed a fireworks-like lung contusion pattern, which resolved within nine days. The authors framed this as a hydrogen explosion injury.
But here’s the reality: this case is a cautionary tale—not definitive proof that pure H₂ or oxyhydrogen inhalation is broadly unsafe and never should be used. The report left out critical details:
- No device specs: There’s no mention of brand, model, flow rates, gas purity, or safety features like flame arrestors, purge vents, or leak detection. Without this information, it’s nearly impossible to know if the problem was the technology itself or simply poor engineering.
- No environmental context: We don’t know the ventilation of the room, the position of the tubing, or how close the heat device was to the cannula. All of these factors strongly affect ignition risk.
- No ignition data: The authors describe an “explosion,” but provide no measurements to distinguish between a small deflagration (fast burn or pop) and a detonation (shock wave). That difference matters.
- Mild outcome: The fact that her bleeding resolved and lung imaging normalized within days suggests this was a limited combustion injury—not a catastrophic blast.
- Speculative causality: The paper phrases it as if hydrogen was definitively to blame, but given the gaps, the connection is suggestive rather than proven.
Perhaps most importantly, this incident reflects negligence and improper protocol more than it does the inherent danger of hydrogen inhalation. Breathing H₂ directly in front of a heat-generating device is a fundamental violation of safe inhalation practice. That is akin to breathing hydrogen gas while smoking a cigarette. That’s Hydrogen Inhalation 101: never pair flammable gases with ignition sources.
So, what’s the takeaway? Use reports like this responsibly. They remind us that hydrogen is flammable and requires respect—but they don’t justify blanket claims that “all non–H₂+air systems are unsafe.” In reality, pure hydrogen and oxyhydrogen devices, when engineered with proper safeguards (flame arrestors, humidity controls, leak detection, internal fans, one way valves, etc ), have been used in numerous human trials—even at high inhaled H₂ concentrations—without systemic combustion injuries.
At H2HUBB, this is exactly why we put devices through basic testing before recommending them. Safety is not defined by whether a system premixes hydrogen with air—it’s defined by how the device is engineered, and how it’s used.
The Takeaways
Hydrogen mixed-with-air devices have carved out a clear niche as arguably the safest and most stable form of H₂ inhalation therapy available today. By keeping hydrogen concentrations well below the flammability threshold, they offer unmatched peace of mind for long-duration users, clinics, and research environments where safety and consistency must come first. For many people, knowing their system will always stay in a safe therapeutic range is a huge advantage.
That said, safety comes with trade-offs. These devices are capped at lower concentrations by design, which means they may not replicate the higher inspired hydrogen levels seen in some of the more aggressive current clinical studies—for example, those in cancer, or other severe disease models. That doesn’t make them ineffective. On the contrary, they provide reliable, clinically relevant doses that can still deliver therapeutic benefits. What it does mean is that they serve a different purpose.
It’s also important to keep perspective: while mixed-air systems are the safest by design, that doesn’t mean other device types—pure H₂ or oxyhydrogen—cannot be used safely. With proper protocols, engineering safeguards, and awareness of their specific risks, these systems can also be operated responsibly at home. The difference is that mixed-air systems “engineer out” almost all of the risk up front, while the others require greater attention to context and safety practices.
In the end, it always comes down to context: who’s using the device, for what condition, and whether maximum dose or maximum safety is the priority in that situation. Mixed-air systems shine when safety and consistency matter most, even if they’re not always the right tool for higher-dose applications. And that’s why at H2HUBB, we test and evaluate across all device types—so you can see both the strengths and limitations clearly, and make the most informed choice for your needs.
Summary and Final Thoughts
Throughout this article, we’ve taken a deep dive into the hydrogen inhalation space — looking at pure H₂ systems, mixed-air devices, and oxyhydrogen (Brown’s gas) units. We’ve explored their strengths, limitations, safety considerations, and misconceptions, and even addressed real-world case reports that sometimes get misused or overstated.
The key takeaway is this: all of these systems have their place. Each form of hydrogen delivery — whether it’s pure H₂, H₂ mixed with air, or oxyhydrogen — can be effective and helpful, depending on the context, the condition, and the preference of the person using it. Some prioritize the highest possible inhaled H₂ concentrations, others value maximum safety, while others look for affordability or simplicity. There isn’t a single “perfect” device for everyone, but there are devices well-suited for different people and different needs.
At H2HUBB, we’re able to address these points objectively because we don’t sell inhalation machines. Our only job is to test them, analyze the data, and provide you with clear guidance on what’s safe, effective, and worth your investment. That independence allows us to say, without bias, that there are multiple good options on the market — and which one is “best” depends more on you than on the device itself.
Now, obviously, we didn’t cover every single angle in this article. If we did, it would be so long that no one would finish reading it. But what we did cover is enough to give you a solid foundation — the kind of framework that helps you think critically about hydrogen inhalation therapy, cut through the marketing hype, and make a decision that you can feel confident about.
In the end, hydrogen inhalation therapy can be done safely and effectively, and the choice of which system to use is less about which one is “better” in absolute terms and more about which one fits your safety preference, your needs, your goals, and your comfort level. That’s the perspective we bring to every evaluation and every recommendation — and it’s why we exist: to make sure people have both confidence and clarity as they bring hydrogen therapy into their lives.
If you’re ready to explore which device is right for you, check out our full list of H2HUBB Approved Hydrogen Inhalation Units Below — tested, safety-vetted, and verified to deliver therapeutic levels of hydrogen gas.

